Weight Loss Program
Weight Loss Program Treatment
Obesity and overweight are serious conditions that can be associated with some of the leading causes of death such as heart disease, stroke and diabetes. Approximately 70% of American adults have obesity or are overweight, and many of those overweight have a weight-related condition. Losing 5% to 10% of body weight through diet and exercise has been associated with a reduced risk of cardiovascular disease in adults with obesity. The research in peptides have reached tremendous mile stones in combating obesity. Peptides such as semaglutide (Ozempic or Wegovy) and tirzepatide (Mounjaro/Zepbound) are a class of drugs called hormone receptor agonists involved in digestion. Semaglutide was approved by the FDA in 2017 initially to lower blood glucose levels in diabetics; however, it was also shown to be very effective in promoting weight loss. In 2020, it was FDA approved for weight management in non-diabetics. The mechanism of action involves appetite suppression, and its efficacy can be sustained through modifications in diet and lifestyle.
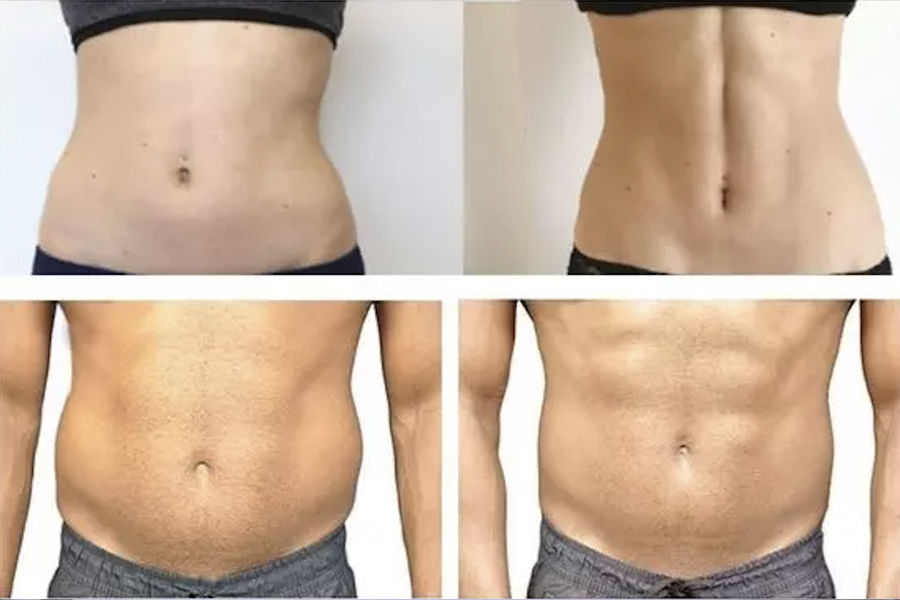
Obesity and overweight are serious conditions that can be associated with some of the leading causes of death such as heart disease, stroke and diabetes. Approximately 70% of American adults have obesity or are overweight, and many of those overweight have a weight-related condition. Losing 5% to 10% of body weight through diet and exercise has been associated with a reduced risk of cardiovascular disease in adults with obesity. The research in peptides have reached tremendous mile stones in combating obesity. Peptides such as semaglutide (Ozempic or Wegovy) and tirzepatide (Mounjaro/Zepbound) are a class of drugs called hormone receptor agonists involved in digestion. Semaglutide was approved by the FDA in 2017 initially to lower blood glucose levels in diabetics; however, it was also shown to be very effective in promoting weight loss. In 2020, it was FDA approved for weight management in non-diabetics. The mechanism of action involves appetite suppression, and its efficacy can be sustained through modifications in diet and lifestyle.
Semaglutide is known as GLP-1 agonist and has been used in treatment for weight loss in non-diabetics for the past 4 years. A recent cohort study found that semaglutide was associated with significant weight loss outcomes compared to placebo, leading to reductions up to 15-20 percent body weight among those patients taking prescription doses of semaglutide. The study was done without any other lifestyle modification or dietary intervention, indicating its effectiveness as a standalone treatment option. The same study also proved its safety for obese and overweight adults without diabetes, deeming it acceptable from both a patient and clinical perspective. Research indicates that semaglutide can effectively make non-diabetic individuals who have difficulty losing excess fat through diet and exercise alone, to lose weight.
Tirzepatide works by activating receptors of hormones secreted from the intestine (GLP-1) as well as glucose-dependent insulinotropic polypeptide (GIP) to both reduce appetite and food intake. Tirzepatide’s effectiveness for chronic weight management (weight reduction and maintenance) in combination with a reduced-calorie diet and increased physical activity was well established in two large randomized, double-blind, placebo-controlled trials. It was subsequently FDA approved for weight management in 2023.
Each individual case is unique, but generally speaking, individuals with a body mass index (BMI) of 30 or higher or a BMI of 27 or higher with at least one weight-related health condition, such as type 2 diabetes, high blood pressure, high cholesterol, or heart disease may qualify for these treatments.
Body mass index (BMI) is a measure of body fat based on height and weight that applies to adult men and women. BMI = Weight (kg)/Height (m squared).
BMI Categories:
Underweight = <18.5
Normal weight = 18.5–24.9
Overweight = 25–29.9
Obesity = BMI of 30 or greater
Patients who have a history of…
- Medullary thyroid cancer
- Multiple endocrine neoplasia
- Pancreatitis
- Retinopathy
- Kidney disease
- Inflammatory bowel disease
- Gallbladder (Liver) disease
- Or, women who are pregnant/breastfeeding or want to become pregnant
- Consultation: An evaluation (in person or virtual) is necessary with our physician to discuss personal and family medical history as well as to establish overall goals of weight loss including diet and exercise.
- Analysis of recent (within 3-6 months) blood work including complete chemistry profile and CBC (blood counts) if indicated.
- Discussion of all potential short and long-term side effects.
- Instructions on self-administration of subcutaneous (under the skin) weekly injections.
- Re-evaluation at 3-6 months for appropriate maintenance dose for the following 6 months.
Semaglutide (Ozempic/Wegovy)
Month Weekly Dose
- 0.25 mg
- 0.50 mg
- 1.00 mg
- 1.70 mg
- 2.40 mg
- 2.40 mg (re-evaluation for maintenance)
Tirzepatide (Mounjaro/Zepbound)
Month Weekly Dose
- 2.5 mg
- 5.0 mg
- 7.5 mg
- 10 mg
- 12.5 mg
- 15 mg (re-evaluation for maintenance)
Although both tirzepatide and semaglutide are used to assist patients with type 2 diabetes and obesity in losing weight and improving cardiovascular diseases, there are some significant distinctions between them.
The main distinctions between these drugs are that they’re two different classes of drugs and have slightly differing targets in the brain.
Tirzepatide is unique in its class of medications since it targets both the GIP and GLP-1 receptor agonists. By activating these receptors, tirzepatide functions differently from semaglutide.
Semaglutide exclusively targets the GLP-1 receptor agonist. One study published in the New England Journal of Medicine (NEJM 2021;385:503-515) showed a slightly superior outcome in diabetics with tirzepatide over semaglutide. However, semaglutide has been on the market longer than tirzepatide and is supported by greater research and clinical proof.
The most common side effect of these weight loss medications is related to the gastrointestinal tract, such as mild to moderate nausea. And, most ease over time of use throughout the weight loss program. Others common potential mild to moderate side effects include vomiting, diarrhea and/or constipation.
Here is a list of other less frequent potential side effects reported in clinical studies:
- anxiety
- bloating
- blurred vision
- chills
- cold sweats
- confusion
- cool, pale skin
- cough
- darkened urine
- depression
- difficulty swallowing
- dizziness
- fast heartbeat
- fever
- headache
- increased hunger
- indigestion
- large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs
- loss of appetite
- nervousness
- nightmare
- pain in the stomach, side, or abdomen, possibly radiating to the back
- seizures
- skin rash
- slurred speech
- tightness in the chest
- trouble breathing
- unusual tiredness or weakness
- yellow eyes or skin
- Acute gallbladder disease
- Acute kidney injury
- Allergic reactions
- Diabetic retinopathy
- Hypoglycemia (when used with insulin or sulfonylurea)
- Increased risk of thyroid tumors
- Pancreatitis
What Client Say
Contact Us
401 Ditmas Ave 2nd Fl,
Brooklyn, NY 11218
We Now Offer Valet Parking!
(718) 618-0058
info@UniqueClinique.com
Sunday: 10:00 AM–6:00 PM
Monday: 10:00 AM–9:00 PM
Tuesday: 10:00 AM–9:00 PM
Wednesday: 10:00 AM–9:00 PM
Thursday: 10:00 AM–9:00 PM
Friday: Hours May Vary
Saturday: Closed
